Can Abiotic Stresses in Plants Be Alleviated by Manganese Nanoparticles or Compounds?
- Review
- Open up Access
- Published:
The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants
Chemic and Biological Technologies in Agriculture volume 4, Article number:v (2017) Cite this article
Abstruse
The apply of bioeffectors, formally known as constitute biostimulants, has become common practise in agriculture and provides a number of benefits in stimulating growth and protecting confronting stress. A biostimulant is loosely defined as an organic fabric and/or microorganism that is applied to enhance nutrient uptake, stimulate growth, enhance stress tolerance or crop quality. This review is intended to provide a broad overview of known effects of biostimulants and their ability to better tolerance to abiotic stresses. Inoculation or awarding of extracts from algae or other plants have benign furnishings on growth and stress adaptation. Algal extracts, protein hydrolysates, humic and fulvic acids, and other compounded mixtures take properties beyond basic nutrition, often enhancing growth and stress tolerance. Non-pathogenic bacteria capable of colonizing roots and the rhizosphere besides have a number of positive effects. These effects include higher yield, enhanced nutrient uptake and utilization, increased photosynthetic activity, and resistance to both biotic and abiotic stresses. While most biostimulants accept numerous and diverse furnishings on plant growth, this review focuses on the bioprotective effects against abiotic stress. Agronomical biostimulants may contribute to make agronomics more sustainable and resilient and offer an alternative to constructed protectants which have increasingly falling out of favour with consumers. An extensive review of the literature shows a clear role for a diverse number of biostimulants that have protective effects against abiotic stress but too reveals the urgent need to address the underlying mechanisms responsible for these furnishings.
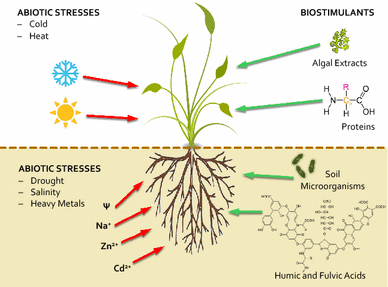
Biostimulants have protective effects against abiotic stress.
Introduction
Plant biostimulants, sometimes referred to as agricultural biostimulants, are a various classification of substances that can be added to the environs around a plant and have positive effects on plant growth and nutrition, just as well on abiotic and biotic stress tolerance. Although about found biostimulants are added to the rhizosphere to facilitate uptake of nutrients, many of these also have protective furnishings against environmental stress such as water arrears, soil salinization and exposure to sub-optimal growth temperatures [1]. Biostimulants are not nutrients per se; instead they facilitate the uptake of nutrients or beneficially contribute to growth promotion or stress resistance [two]. A newly emerged paradigm emphasizes that plants are not standalone entities within their environments; instead they are host and partner to microorganisms of leaner and fungi; plants are a host to numerous microbiota and those associations, both outside and within its tissues, allow them to respond and conform to abiotic and biotic stress [3]. Reasonably, if nosotros functionally optimize these associations, we may strengthen their role in plant stress protection.
The manufacture definition of biostimulants was originally proposed in 2012 and stated: "Establish biostimulants contain substance(s) and/or microorganisms whose function when applied to plants or the rhizosphere is to stimulate natural processes to raise/benefit nutrient uptake, nutrient efficiency, tolerance to abiotic stress, and crop quality. Biostimulants have no straight action confronting pests, and therefore exercise not autumn within the regulatory framework of pesticides". Biostimulants were loosely divers for a long time and often regarded dubiously considering of their aggregate nature and the inherent difficulty to determine which specific components were making beneficial contributions. The definition proposed past du Jardin [1] "A plant biostimulant is whatsoever substance or microorganism applied to plants with the aim to enhance nutrition efficiency, abiotic stress tolerance and/or crop quality traits, regardless of its nutrients content" represents the clearest and virtually curtailed mode to define biostimulants.
Our agreement of biostimulants and their potential effects has been expanding at a considerable rate [4]. The function of biostimulants, specifically in regard to growth promotion and food availability, has been reviewed (du Jardin [i, 4–6]). In improver to numerous general reviews, many categories of specific biostimulants accept been extensively reviewed such every bit protein hydrolysates [7], seaweed extracts [8], silicon [9], chitosan [10], humic and fulvic acids [11], the role of phosphite [12], arbuscular mycorrhizal fungi [13], trichoderma [fourteen], plant growth-promoting rhizobacteria [15]. These reviews have focused on found growth promotion and biotic stress but our intent with this review is to comprehensively address what is known about biostimulants ameliorating the effects of abiotic stress (Tabular array 1). The majority of these studies were conducted every bit greenhouse or field experiments. The literature has mainly focused on crop species with a large representation of cereal crops such as wheat, barley, and corn. Finally, nosotros attempted to map different categories vs. their physiological office in plants.
Algal extracts
Seaweed extracts (SWE) equally biostimulants are emerging as commercial formulations for use as plant growth-promoting factors and a method to improve tolerance to salinity, heat, and drought. Algal extracts target a number of pathways to increment tolerance nether stress (Fig. one). Seaweeds are crimson, dark-green, and brownish macroalgae that represent ten% of marine productivity [8]. Macroalgae take been used as organic fertilizers for thousands of years and are all the same in use [64]. Currently, there are over 47 companies producing and marketing various algal extracts for agricultural use; the majority of the formulations are from the brown algae, Ascophyllum nodosum [65].

Summary of main key mechanisms targeted by algal-based biostimulants
While the growth-promoting effects of seaweed extracts have been documented in many species [8, 66], very little is really known about the mechanisms behind these effects. The variable and complex nature of these substances makes it difficult to determine exactly which components are playing a primal function. Commercial formulations of SWEs are often proprietary, and the composition is largely dependent on the method of extraction. Indeed, label of the bodily composition of almost common algal-based commercial products would exist useful commencement step to better hypothesize and/or draw a crusade–effect relationship of their mechanism of action. Mechanical disruption, powder, acid or alkali extractions are some of the more common methods employed [8]. Nearly commercial products are derived from carmine (ex Lithothamnium calcareum) and chocolate-brown (ex Ascophyllum nodosum, Durvillaea potatorum) macroalgae [67]. The part of SWEs and common cold tolerance is now emerging. Very recent work has focused on SWEs and their ability to enhance tolerance to chilling stress. When multiple extracts were tested for their ability to enhance cold tolerance in maize only extracts rich in Zn and Mn were able to enhance tolerance through enhanced ROS responses. In this case, the protective effects likely stem from supplying plants with micronutrients that play a role every bit co-factors in anti-oxidative enzymes [63]. These results bespeak that nutrient deficiency stress induced by cold tin be overcome past supplying SWEs rich in micronutrients to amend oxidative stress tolerance. Previous studies with corn seedlings nether root chilling stress supplemented with micronutrients demonstrated the utility of nutrient seed priming [68].
Some work has been washed in model systems with the goal of determining the physiological and molecular responses induced by SWEs. In guild to better understand the active components of A. nodosum, Rayirath et al. [55] separated the organic-sub-fractions of extracts and tested them with Arabidopsis thaliana and freezing experiments. Plants grown in vitro with sub-fractions added to the substrate or in "Peat pellet freezing assays" irrigated with sub-fractions were tested for freezing tolerance. The authors found that the ethyl acetate extracted fraction, rich in fat acids and sterols enhanced freezing tolerance over water treated (controls) at temperatures from −2.5 to −5.5 °C. Treated plants maintained faster rates of recovery, greater membrane integrity, and had 70% less chlorophyll damage upon freezing recovery as well every bit increased expression of key freezing tolerance genes such as RD29A, COR15A, and CBF3 [55]. Priming of fundamental tolerance genes prior to exposure to stress greatly increases tolerance in many cases. The lipophilic components were found to be rich in fatty acids such as butyric acid, palmitic acrid, oleic acrid, linoleic acid the sterol fucosterol. These extracts increased proline content and total soluble sugars, contributing to freezing tolerance [56]. A. nodosum extracts have even been used to reduce cold stress sensitivity in Kappaphycus alvarezii. Kappaphycus alvarezii is a red algae and the most important source of carrageenans; which are hydrophilic colloids largely used in foods and dairy products [29, 69]. Algal extracts have also been used on Kentucky bluegrass (Poa pratensis L. cv. Costly) to alleviate salinity stress from saline watering in turfgrass experiments [57]. Similarly SWE-based cytokinins accept been used on creeping bentgrass (Agrostis stolonifera Fifty.) to ameliorate tolerance to oestrus stress [58]. SWEs from A. nodosum have as well been used for ornamental plants, such equally Spiraea nipponica "Snowmound" and Pittosporum eugenioides "Variegatum", to enhance drought tolerance. Treated plants showed college phenolic, proline, and flavonoid content while demonstrating improved physiology under balmy drought stress atmospheric condition [62].
In horticultural crops and trees, SWE have been largely used for similar purposes. A. nodosum SWE increased RWC, Fresh Weight, and Dry Weight in spinach (Spinacia oleracea 50.) plants under drought stress with some agin effects on the nutritional value through reduced ferrous ion chelating ability [59]. SWE applied to seedlings of lettuce (Lactuca sativa L.) enhanced cotyledon growth like to fertilization with potassium [lx].
Foliar application of marine bioactive substances (isopropanol extracts from microalgae) to grape plants (Vitis vinifera L) increased leaf water potential and stomatal conductance under drought stress [61]. Consistent with an improved stomatal response, it was also observed that K+ and Catwo+ fluxes at the stomatal level were higher in treated plants. Commercial formulations of A. nodosum have been tested on almond plants (Prunus dulcis [Mill.] D. A. Webb), which demonstrated increased growth and accumulation of 1000+. In weather with ample K+ both MegaFol and GroZyme (Valagro, Atessa, Chieti, ITALY) increased leaf surface area and number of leaves greater than controls treated with h2o or K+. In K+-deficiency conditions but MegaFol and a foliar awarding of K+ was able to stimulate growth, although at lower levels than observed with adequate 1000+ nutrition [30]. Accumulation of K+ is an essential step in protecting against both ionic and osmotic stress and may contribute to tolerance. Orange trees, Citrus sinensis Fifty., subjected to drought stress and treated with commercial extracts of A. nodosum had better h2o relations and increased water utilize efficiency (WUE) nether irrigation at 50% restitution of evapotranspired h2o [31]. The hope of biostimulants to increase drought tolerance and WUE holds bully potential for drought prone regions where horticultural crops and fruit trees are agronomically important but water availability is becoming less reliable due to urbanization and climate change.
Equally before noted, well-nigh all of the above-mentioned experiments with SWE apply commercial formulations. This may be of some business organization, due to the variable nature of these products and conception methods. A recent transcriptomic written report using A. thaliana plants treated with two different commercial A. nodosum extracts showed that not all extracts are akin. One commercial product resulted in dysregulation of 4.47% of the transcriptome while the other extract only affected 0.87% [seventy]. Since transcriptional priming is probable a fundamental component in enhancing abiotic stress tolerance using SWEs, these differences imply significant variability in responses elicited. Compositions of the extracts differed profoundly, indicating that option of commercial production may have a significant event on constitute responses. Commercial formulations are ofttimes proprietary and the exact composition and extraction methods, shifting the burden to the research community to analyse and isolate the active components in these products. In lodge to identify and characterize how these SWEs impact plants, some form of standardization is necessary.
Carbohydrates, proteins, amino acids, and lipids
Protein hydrolysates are mixtures of polypeptides, oligopeptides, and free amino acids derived from fractional hydrolysis of agricultural past-products from animals and plants [7]. Carbohydrates, proteins, amino acids, and lipids may increase stress tolerance through unlike (Fig. 2). The effects of amino acids on ion fluxes across membranes have been conspicuously established, with most having a positive effect on reducing NaCl-induced potassium efflux [48]. Protein hydrolysates (PH) are oftentimes sold as formulations that include plant growth regulators. The bulk of PH products, over ninety%, are produced from chemical hydrolysis of creature by-products while enzymatically processed plant-based products are a recent evolution [seven].
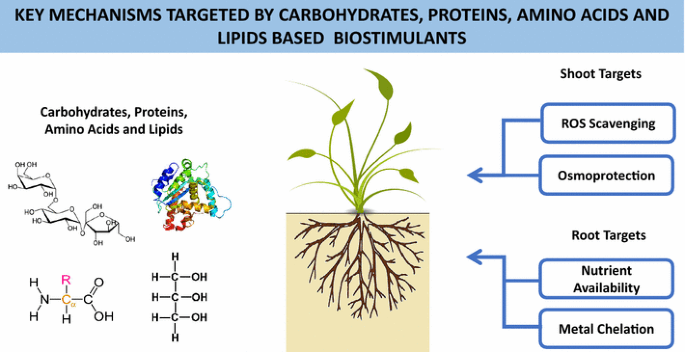
Summary of main key mechanisms targeted by saccharide-, protein-, amino acrid-, and lipid-based biostimulants
Megafol (Valagro, Atessa, Chieti, ITALY) is a commercial biostimulant comprising vitamins, amino acids, proteins, and betaines from plant and algal extracts. Application of Megafol to love apple plants under drought stress enhanced induction of a number of drought responsive genes such as tomato orthologs of RAB18 and RD29B. Treated plants also had higher fresh weight and relative water content under drought stress, indicating a protective effect on water status and stress responsive genes [43, 71]. When hydrolysate-based biostimulants from alfalfa (Medicago sativa L.), containing triacontanol (TRIA) and indole-3-acerb acid (IAA), were applied to maize plants under salt stress, the protective effects were amplified. Treated plants had higher flavonoid, proline, and potassium content in salt stress conditions over untreated controls [49]. Extracts that are rich in amino acids may play a role in increasing cold tolerance. When lettuce plants (Lactuca sativa) were treated with an amino acid mixture, derived from enzymatic hydrolysis of proteins, (Terra-Sorb) and subjected to cold, treated plants had higher fresh weights and improved stomatal conductance [51]. Utilize of animal derived amino acid hydrolysates on strawberry plants after transplantation and cold stress did not improve survival though some growth promotions were observed in the absence of stress [72]. Perennial Rye-grass (Lolium perenne L.) treated with hydrolyzed amino acids and loftier temperatures (36 °C) had improved photosynthetic efficiency over command plants [51].
Mutants of A. thaliana scarce in production of proline take stress sensitive phenotypes [73]. These plants tin accept their phenotype rescued with exogenous awarding of l-proline, a common amino acid available in biostimulant formulations of various amino acids and hydrolysate mixtures [74]. Hydrolysates from wheat germs testify strong anti-oxidant and free radical scavenging properties likewise as the ability to chelate some metals [50].
Lettuce (Lactuca sativa L.) is especially salt sensitive and the addition of institute-derived protein hydrolysates improved fresh yield, dry out biomass, and root dry out weight likewise as increased concentrations of osmoyltes, glucosinolates and the composition of sterols and terpenes [52]. Hydrolysates accept applications for trees, which require considerable investment costs and can be vulnerable to drought. Japanese persimmon trees, Diospyros kaki L. cv. "Rojo Brillante" grafted on Diospyros lotus Fifty., are particularly sensitive to drought stress [53]. Treatment of these copse with calcium protein hydrolysates decreased chloride uptake under saline irrigation, lowered h2o potentials every bit well as increased the concentration of uniform solutes [53], all of which would enhance constitute growth under saline stress.
Recent reports indicate that melatonin, derived from l-tryptophan via the shikimate pathway, can prime seeds to tolerate agin environmental atmospheric condition at imbibition and germination stages [75]. Corn seeds pre-treated with melatonin testify increased tolerance to chilling stress upon germination, indicating a priming effect by melatonin [44]. Melatonin may prove to be an effective biostimulant for improving stress tolerance of seedlings.
Glycinebetaine is a compatible solute accumulated in many plants in response to salt stress [76]. Exogenous awarding of glycinebetaine has increased tolerance for ecology stresses such equally drought, chilling, freezing, salinity, and oxidative stress. Foliar awarding of glycinebetaine results in rapid uptake past leaves and concentration in meristematic tissues. Rapid uptake and localization of glycinebetaine in these well-nigh vulnerable tissues are particularly beneficial in chilling and freezing stress where glycinebetaine can exert a protective issue [77]. Transgenic plants of various species expressing two biosynthetic genes, codA and betA, produce more glycinebetaine and had an increased tolerance to abiotic stress [38, 78]. Exogenous application of small amounts of uniform solutes such every bit proline and betaine to barley roots resulted in an immediate reduction of NaCl-induced efflux of K+, indicating that ion fluxes beyond the membrane can be affected by relatively low concentrations of compatible solutes [79]. The cause–result relationship between aggregating of compatible solutes and stress protection still remains to be fully understood [eighty]. Withal, a improve understanding of the specific mechanisms of action of these molecules is condign increasingly important if we want to brand predictions on which combination of biostimulants tin can exist more effective.
Humic and fulvic acids
Humic and fulvic substances are the major organic components of lignites, soil, and peat. Humic and fulvic acids are produced by the biodegradation of organic matter resulting in a mixture of acids containing phenolate and carboxyl groups. Fulvic acids are humic acids with a college oxygen content and lower molecular weight [81]. A number of examples exist indicating the potential for these substances to improve abiotic stress tolerance in plants (Fig. 3). Pre-treatment of tall fescue (Festuca arundinacea Schreb.) and creeping bentgrass (Agrostis palustris Huds. A.) with seaweed extract and humic acrid increased leaf hydration under dry soil weather as well as root growth, shoot growth, and antioxidant capacity [35, 58]. Further studies with bentgrass showed these extracts, high in cytokinins, combined with humic acrid increased drought tolerance also as endogenous cytokinin content [37].
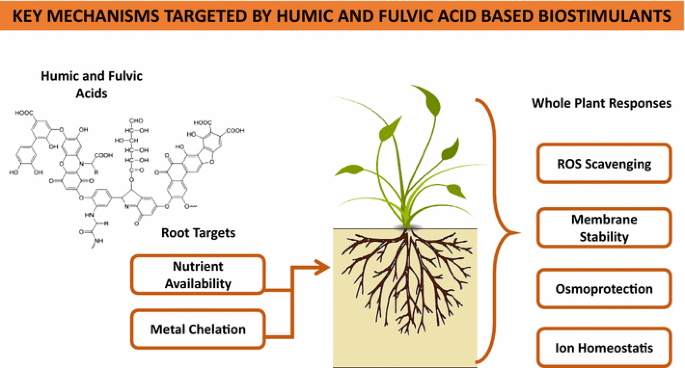
Summary of main key mechanisms targeted by humic- and fulvic acid-based biostimulants
Handling of bell pepper (Capsicum annuum L. cv. Demre) with humic acid and phosphorous resulted in plants with reduced Na content and elevated N, P, K, Ca, Fe, Mg, S, Mn, and Cu ion contents in roots and shoots, which were associated with a full general protective effect under balmy salinity stress [twoscore]. Application of humic acids to common edible bean (Phaseolus vulgaris Fifty.) nether high salinity (120 mM NaCl) increased endogenous proline levels and reduced membrane leakage [42], which are both indicators of ameliorate adaptation to saline envirnoments.
Humic acrid extracts seem to be benign also for field crop monocots. Extracts from vermicompost practical to rice (Oryza sativa 50.) played a role in activating anti-oxidative enzymatic office and increased ROS scavenging enzymes. These enzymes are required to inactivate toxic-complimentary oxygen radicals produced in plants nether drought and saline stress [41]. One possible mode of action for vermicompost may be the differential regulation of proton ATPases located in the vacuolar and plasma membranes. When Micro-Tom tomato plants were treated with vermicompost, plasma membrane proton extrusion was increased past over 40% which facilitated acid growth and nutrient uptake potential. Interestingly, the auxin insensitive mutant diageotropica (dgt) showed no increment in proton extrusion, indicating that humic substance may increment root growth through mediating auxin signalling [82].
Microorganisms affecting stress tolerance
While plants are known to establish symbiotic relationships with leaner, our understanding of those relationships under abiotic stress is rudimentary. Withal, some of the targets of microorganisms that increase abiotic stress tolerance accept been identified (Fig. 4). Bacteria with the potential to act equally biostimulants have been isolated from a number of ecosystems with saline, alkaline, acidic, and arid soils. These bacteria belong to several genera such as Rhizobium, Bradyrhizobium, Azotobacter, Azospirillum, Pseudomonas, and Bacillus. Members of these genera have developed strategies to suit and thrive under adverse weather condition [83, 84]. Amongst these adaptations, alterations to the composition of the jail cell wall and the ability to accrue high concentrations of soluble solutes are common. These allow for enhanced water retention and increased tolerance to osmotic and ionic stress. Jail cell wall composition is altered through enrichment for exopolysaccharides (EPS) and lipopolysaccharide–proteins and polysaccharide–lipids which my form a protective biofilm on the root surface [85, 86]. Plant growth-promoting rhizobacteria (PGPR) inoculated soils can better plant abiotic stress responses. A number of contempo reviews have extensively covered the protective effects of Rhizobium confronting abiotic stress in plants [87]. About documented growth enhancement adamant past these leaner is associated with high level of IAA, which has been proven to convalesce salt stress [88] and EPS production that may help in maintaining a film of hydration around the roots and/or assist re-establishing favourable water potential gradients under water limitations. These functions accept been proven useful under saline stress [89], extremes of temperature, pH, salinity, and drought [87, ninety]. Inoculation of maize with Azotobacter strains has been shown to take general positive furnishings under saline stress past facilitating uptake of K+ and exclusion of Na+ every bit well as increasing phosphorous and nitrogen availability [24]. In wheat, inoculation of common salt tolerance Azobacter strains increased biomass, nitrogen content, and grain yield under table salt stress [25].
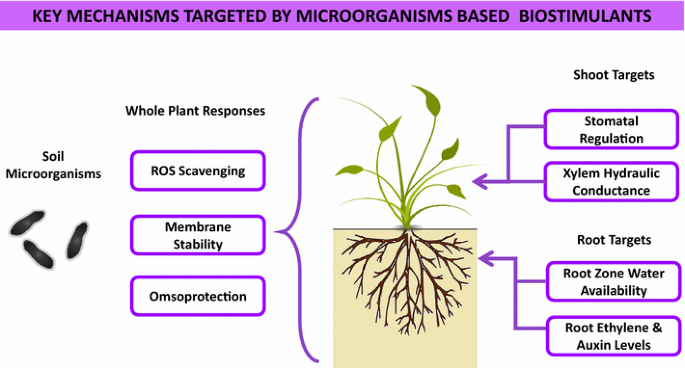
Summary of main key mechanisms targeted by microorganism-based biostimulants
Tolerance to salt stress varies within these microorganisms and their tolerance can confer advantages to the host relationship under stress weather condition. When two legumes, pea (Pisum sativum) and fava bean (Vicia faba), were inoculated with 2 different strains of Rhizobium leguminosarum, a salt-tolerant (GRA19) and salt-sensitive (GRL19) plants inoculated with the common salt-tolerant strain performed better under moderate table salt stress [54]. The authors further found that pea plants had larger nodules and high levels of nitrogen fixation under salt stress when inoculated with GRA19, the salt-tolerant strain of R. leguminosarum. Similar results have been observed for not-symbiotic free-living soil leaner that are capable of fixing nitrogen. Azospirillum brasilense is closely associated with the institute rhizosphere and can colonize the surface of roots. When chickpea (Cicer arietinum L.) and faba bean were inoculated with A. brasilense, they experienced enhanced nodulation by native rhizobia and greater tolerance to salt stress [xviii]. Some other free-living nitrogen-fixing species, Azotobacter chrococcum A2 demonstrated salt tolerance. Inoculation with A. chrococcum has been shown to increase yields of pea, tater, rice, wheat, and cotton in saline-barren soils. Increased root length and shoot growth was likewise observed with inoculation [26, 27] with meaning positive yield effects for wheat (from 2.8 to iii.five t ha−ane when grown in conjunction with A. chrococcum) [26, 27].
In barley, Hartmannibacter diazotrophicus E19 (T) is capable of colonizing roots in saline atmospheric condition. Inoculation of roots in saline soil increased root and shoot mass significantly, 308 and 189%, respectively. Inoculated roots also had increased relative water content over iii and a half times that of control plants [39]. Loftier concentrations of salt tin also be inhibitory to rhizobial bacteria. While certain strains of R. leguminosarum, such as viciae SAAN1, are very common salt tolerant and able to withstand up to 0.34 M NaCl, they often bear witness lower rates of nodulation in saline soils. These strains are often less competitive with natural rhizobial populations, however.
The stress protection of bacterial biostimulants to rainfed field crops can exist of particular relevance nether increasing temperatures foreseen past most climatic change prediction models. Wheat inoculated with the thermo tolerant Pseudomonas putida strain AKMP7 significantly increased heat tolerance. Inoculated plants had increased biomass, shoot and root length, and seed size. ROS generation under stress handling was also lessened, with lower levels of expression observed for ROS response genes such every bit superoxide dismutase, ascorbate peroxidase, and catalase [45]. Like results have been institute with sorghum and other Pseudomonas putida AKMP strains [46]. Psychrophilic (cold-adapted) microorganisms are capable of surviving in extreme conditions and their interactions with plants provide potential mechanisms for improving tolerance [91]. While many strains of soil leaner with growth-promoting properties have been isolated from low-temperature conditions, few have been tested in conjunction with plants subjected to cold stress.
Wheat inoculated with the cold-tolerant plant growth-promoting leaner Pantoea dispersa showed improved growth and nutrient uptake, probable due to the solublization of phosphorous and product of IAA [47].
Inoculation of soil with psychrotolerant (common cold tolerant) leaner can play a function in chilling tolerance. The psychrotolerant soil bacterium, Burkholderia phytofirman, is a plant-growth-promoting rhizobacterium (PGPR) that is capable of colonizing multiple constitute species. B. phytofirman was shown to play a role in enhancing spooky tolerance in Vitis vinifera L. past increasing ROS scavenging metabolites and stress-induced genes. Inoculated plants also recovered faster from spooky stress, returning to normal metabolic levels more quickly than controls [33]. B. phytofirman inoculation also alters carbohydrate metabolism and aggregating while having a protective result on net photosynthesis during cold acclimation and stress [32, 92].
Tomato plants (Solanum lycopersicum cv Mill) were inoculated with common cold-tolerant strains of Pseudomonas vancouverensis, and frederiksbergensis likewise every bit Flavobacterium glaciei that were isolated from agronomical fields during winter. Treated tomato seedlings were subjected to a week of chilling stress at 15C and inoculation iii of these strains showed significantly reduced electrolyte leakage and ROS activity [34]. Improved stress tolerance and growth-promoting furnishings of microorganism treatments have been seen in other species too. Inoculation of lettuce (Lactuca sativa L., cv Mantecosa) seeds with A. brasilense increased germination in the presence of salt and demonstrated tolerance through college total fresh and dry weights of plants at harvest [19]. Additional experiments studying these effects take shown increased biomass, chlorophyll, ascorbic acid content, antioxidant content, and mail service-harvest shelf life after beingness subjected to common salt stress [20]. Sweet pepper (Capsicum annuum L.) inoculated with A. brasilense and Pantoea dispersa was not afflicted past moderate levels of salinization, up to eighty mM NaCl, while uninoculated control plants demonstrated lower DW starting at 40 mM NaCl [23].
Triticum aestivum cv. Buck Ombú inoculated with A. brasilense sp. 245 and subjected to common salt stress (320 mM NaCl) and osmotic stress (twenty and 30% PEG 6000) had college FW, DW, and RWC than non-inoculated controls [21]. Assay of phospholipids and fatty acrid composition in inoculated wheat indicated that the distribution profiles of major root phospholipids are altered in inoculated plants, peradventure contributing to the increased tolerance [xvi]. Wheat inoculated with Azospirillum lipoferum and irrigated with eighty mM NaCl had significantly college foliage and root dry weight than uninoculated controls [28].
While the mechanisms by which A. brasilense confer tolerance to osmotic stress are not clear, some testify indicates that inoculation induces wider xylem vessels and greater hydraulic conductance [17]. In inoculated tomato plants subjected to water stress similar changes take been observed, such equally larger xylem vessel surface area, college stem-specific hydraulic electrical conductivity, thicker stems [22]. Pepper plants co-inoculated with A. brasilense and Pantoea dispersa accumulated more dry thing under salt stress. Inoculated plants showed higher stomatal conductance and rates of photosynthesis under salt stress. The chlorophyll concentration and efficiency of photosystem Two were not affected in inoculated plants under stress conditions [23].
Inhibition of root growth under common salt stress conditions is well documented. One of the primary causes of this inhibition is the product and perception of ethylene in the roots [93]. Plants and PGPR both have ACC-deaminases, which possess the ability to lower the concentration of ethylene in the roots and root zone. PGPR-derived ACC-deaminases tin reduce ethylene induced inhibition by reducing root zone ethylene [94] and contribute to maintain relatively college root-to-shoot ration, a trait that would upshot beneficial under water shortage.
Conclusions
Biostimulant treatments of agricultural crops have the potential to amend constitute resilience to environmental perturbations. In club to fine-melody awarding rates, biostimulant-institute specificities and techniques is identified that may yield highest impact on stress protection; high priority should exist given to better agreement of the causal/functional mechanism of biostimulants. Only once a good agreement of these mechanisms has been reached; we volition be able to motility to the next generation of biostimulants where synergies and complementary mechanisms tin be functionally designed. Comprehension of the specific mechanisms that should be potentiated to overcame a specific stress can be based today on sound/reasonable hypotheses and exist more than fruitful than the effort-and-encounter approach. A comprehensive and systematic approach has been proposed to detect and narrate novel biostimulants and sympathise the mode of action for those both known and new using a combined approach utilizing biology, chemistry, and 'omics [95]. Meta-analysis of the furnishings of biostimulants has been proposed and an extensive meta-level examination of humic substances on plant growth has been conducted. The analysis found that humic substances increased the dry weight of shoots and roots by at least xx% [96]. Withal, it should be noted that the diverse weather condition, compositions, and species tested practice not lend to robust meta-level analysis when an excessive number of variables is present. Identification of synergistic/complementary properties of biostimulants can be pivotal to develop specific formulations targeted to enhance plant response to abiotic stress. For instance, biostimulants for improving found resilience in water limiting environments should stimulate root vs. shoot growth which would let plants to explore deeper soil layer during the drought season and stimulate the synthesis of compatible solutes to re-establish favourable water potential gradients and water uptake at diminishing soil water. Like positive effects can be given by those microbial biostimulants that create absorption surfaces around the root systems and sequester soil water in favour of the plants.
References
-
du Jardin P. Institute biostimulants: definition, concept, main categories and regulation. Sci Hortic. 2015;30(196):iii–14.
-
Brownish P, Saa South. Biostimulants in agriculture. Front Plant Sci [Internet]. 2015;six. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4550782/. Accessed 13 October 2015.
-
Vandenkoornhuyse P, Quaiser A, Duhamel M, Le Van A, Dufresne A. The importance of the microbiome of the plant holobiont. New Phytol. 2015;206(4):1196–206.
-
Colla Grand, Rouphael Y. Biostimulants in horticulture. Sci Hortic. 2015;xxx(196):1–ii.
-
Du Jardin, P. The science of plant biostimulants—a bibliographic analysis. Ad hoc Report Written report to the European Commission DG ENTR; 2012.
-
Calvo P, Nelson L, Kloepper JW. Agronomical uses of plant biostimulants. Establish Soil. 2014;383(1–2):three–41.
-
Colla Thousand, Nardi Southward, Cardarelli M, Ertani A, Lucini Fifty, Canaguier R, et al. Protein hydrolysates as biostimulants in horticulture. Sci Hortic. 2015;30(196):28–38.
-
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B. Seaweed extracts as biostimulants in horticulture. Sci Hortic. 2015;xxx(196):39–48.
-
Savvas D, Ntatsi G. Biostimulant action of silicon in horticulture. Sci Hortic. 2015;30(196):66–81.
-
Pichyangkura R, Chadchawan S. Biostimulant action of chitosan in horticulture. Sci Hortic. 2015;196:49–65.
-
Canellas LP, Olivares FL, Aguiar NO, Jones DL, Nebbioso A, Mazzei P, et al. Humic and fulvic acids as biostimulants in horticulture. Sci Hortic. 2015;30(196):fifteen–27.
-
Gómez-Merino FC, Trejo-Téllez LI. Biostimulant activity of phosphite in horticulture. Sci Hortic. 2015;196:82–90.
-
Rouphael Y, Franken P, Schneider C, Schwarz D, Giovannetti G, Agnolucci Yard, et al. Arbuscular mycorrhizal fungi human action as biostimulants in horticultural crops. Sci Hortic. 2015;30(196):91–108.
-
López-Bucio J, Pelagio-Flores R, Herrera-Estrella A. Trichoderma as biostimulant: exploiting the multilevel properties of a plant benign mucus. Sci Hortic. 2015;xxx(196):109–23.
-
Ruzzi Yard, Aroca R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci Hortic. 2015;30(196):124–34.
-
Pereyra MA, Zalazar CA, Barassi CA. Root phospholipids in Azospirillum-inoculated wheat seedlings exposed to water stress. Found Physiol Biochem. 2006;44(11–12):873–9.
-
Pereyra MA, García P, Colabelli MN, Barassi CA, Creus CM. A better h2o status in wheat seedlings induced by Azospirillum under osmotic stress is related to morphological changes in xylem vessels of the coleoptile. Appl Soil Ecol. 2012;53(1):94–seven.
-
Hamaoui B, Abbadi J, Burdman S, Rashid A, Sarig South, Okon Y. Effects of inoculation with Azospirillum brasilense on chickpeas (Cicer arietinum) and faba beans (Vicia faba) under unlike growth conditions. Agronomie. 2001;21(half dozen–7):553–60.
-
Barassi CA, Ayrault G, Creus CM, Sueldo RJ, Sobrero MT. Seed inoculation with Azospirillum mitigates NaCl effects on lettuce. Sci Hortic. 2006;109(one):viii–fourteen.
-
Fasciglione G, Casanovas EM, Quillehauquy Five, Yommi AK, Goñi MG, Roura SI, et al. Azospirillum inoculation effects on growth, production quality and storage life of lettuce plants grown nether salt stress. Sci Hortic. 2015;195:154–62.
-
Creus CM, Sueldo RJ, Barassi CA. Shoot growth and h2o condition in Azospirillum-inoculated wheat seedlings grown nether osmotic and salt stresses. Plant Physiol Biochem. 1997;35(12):939–44.
-
Romero AM, Vega D, Correa Os. Azospirillum brasilense mitigates water stress imposed by a vascular affliction by increasing xylem vessel surface area and stem hydraulic conductivity in tomato. Appl Soil Ecol. 2014;82:38–43.
-
Del Amor FM, Cuadra-Crespo P. Plant growth-promoting leaner equally a tool to meliorate salinity tolerance in sweet pepper. Funct Plant Biol. 2012;39(1):82–90.
-
Rojas-Tapias D, Moreno-Galván A, Pardo-Díaz S, Obando One thousand, Rivera D, Bonilla R. Upshot of inoculation with plant growth-promoting leaner (PGPB) on amelioration of saline stress in maize (Zea mays). Appl Soil Ecol. 2012;61:264–72.
-
Chaudhary D, Narula N, Sindhu SS, Behl RK. Plant growth stimulation of wheat (Triticum aestivum Fifty.) by inoculation of salinity tolerant Azotobacter strains. Physiol Mol Biol Plants. 2013;xix(4):515–9.
-
Egamberdiyeva D, Höflich Yard. Influence of growth-promoting leaner on the growth of wheat in different soils and temperatures. Soil Biol Biochem. 2003;35(7):973–8.
-
Egamberdiyeva D, Höflich Thousand. Effect of constitute growth-promoting bacteria on growth and nutrient uptake of cotton fiber and pea in a semi-arid region of Uzbekistan. J Arid Environ. 2004;56(two):293–301.
-
Bacilio M, Rodriguez H, Moreno M, Hernandez J-P, Bashan Y. Mitigation of salt stress in wheat seedlings by a gfp-tagged Azospirillum lipoferum. Biol Fertil Soils. 2004;40(3):188–93.
-
Loureiro RR, Reis RP, Marroig RG. Effect of the commercial extract of the dark-brown alga Ascophyllum nodosum Mont. on Kappaphycus alvarezii (Doty) Doty ex P.C. Silva in situ submitted to lethal temperatures. J Appl Phycol. 2014;26(ane):629–34.
-
Saa South, Olivos-Del Rio A, Castro South, Brown PH. Foliar application of microbial and found based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Factory.] D. A. Webb). Front. Plant Sci. 2015;half-dozen:87.
-
Spann TM, Little HA. Applications of a commercial extract of the brownish seaweed Ascophyllum nodosum increases drought tolerance in container-grown "hamlin" sweetness orangish nursery trees. Hort Scientific discipline. 2011;46(iv):577–82.
-
Fernandez O, Theocharis A, Bordiec S, Feil R, Jacquens L, Clément C, et al. Burkholderia phytofirmans PsJN acclimates grapevine to cold by modulating carbohydrate metabolism. MPMI. 2012;25(four):496–504.
-
Theocharis A, Bordiec S, Fernandez O, Paquis Due south, Dhondt-Cordelier S, Baillieul F, et al. Burkholderia phytofirmans PsJN Primes Vitis vinifera L. and Confers a meliorate tolerance to low nonfreezing temperatures. MPMI. 2012;25(ii):241–ix.
-
Subramanian P, Kim One thousand, Krishnamoorthy R, Mageswari A, Selvakumar Thousand, Sa T. Cold stress tolerance in psychrotolerant soil leaner and their conferred chilling resistance in tomato (Solanum lycopersicum Mill.) under low temperatures. PLoS Ane. 2016;11(8):e0161592.
-
Zhang 10, Schmidt RE, Ervin EH, Doak S. Creeping bentgrass physiological responses to natural plant growth regulators and atomic number 26 nether two regimes. HortScience. 2002;37(half dozen):898–902.
-
Zhang X, Schmidt RE. Hormone-containing products'; impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Sci. 2000;40(5):1344–9.
-
Zhang X, Ervin EH. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Ingather Sci. 2004;44(5):1737.
-
Park E-J, Jeknić Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata Northward, et al. Genetic engineering of glycinebetaine synthesis in tomato plant protects seeds, plants, and flowers from chilling harm. Found J. 2004;twoscore(4):474–87.
-
Suarez C, Cardinale Yard, Ratering S, Steffens D, Jung South, Montoya AMZ, et al. Constitute growth-promoting effects of Hartmannibacter diazotrophicus on summer barley (Hordeum vulgare L.) nether salt stress. Appl Soil Ecol. 2015;95:23–30.
-
Çimrin KM, Türkmen Ö, Turan One thousand, Tuncer B. Phosphorus and humic acid application alleviate salinity stress of pepper seedling. Afr J Biotechnol [Internet]. 2010;9(36). http://www.ajol.info/index.php/ajb/article/view/92903. Accessed 16 Nov 2015.
-
García AC, Santos LA, Izquierdo FG, Sperandio MVL, Castro RN, Berbara RLL. Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol Eng. 2012;47:203–8.
-
Aydin A, Kant C, Turan 1000. Humic acid application alleviates salinity stress of bean (Phaseolus vulgaris Fifty.) plants decreasing membrane leakage. Afr J Agric Res. 2012;7(seven):1073–86.
-
Petrozza A, Santaniello A, Summerer S, Di Tommaso G, Di Tommaso D, Paparelli Eastward, et al. Physiological responses to Megafol® treatments in tomato plants under drought stress: a phenomic and molecular approach. Sci Hortic. 2014;22(174):185–92.
-
Kołodziejczyk I, Dzitko K, Szewczyk R, Posmyk MM. Exogenous melatonin improves corn (Zea mays Fifty.) embryo proteome in seeds subjected to chilling stress. J Plant Physiol. 2016;ane(193):47–56.
-
Ali SKZ, Sandhya V, Grover M, Linga VR, Bandi V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) nether heat stress. J Constitute Interact. 2011;6(iv):239–46.
-
Ali SKZ, Sandhya V, Grover M, Kishore N, Rao LV, Venkateswarlu B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol Fertil Soils. 2009;46:45–55.
-
Selvakumar G, Kundu S, Joshi P, Nazim S, Gupta AD, Mishra PK, et al. Label of a cold-tolerant plant growth-promoting bacterium Pantoea dispersa 1A isolated from a sub-tall soil in the North Western Indian Himalayas. World J Microbiol Biotechnol. 2008;24(seven):955–lx.
-
Cuin TA, Shabala S. Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta. 2007;225(three):753–61.
-
Ertani A, Schiavon M, Muscolo A, Nardi Southward. Alfalfa plant-derived biostimulant stimulate short-term growth of common salt stressed Zea mays L. plants. Institute Soil. 2012;364(1–2):145–58.
-
Zhu M, Zhou H, Qian H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006;41(6):1296–302.
-
Botta A. Enhancing institute tolerance to temperature stress with amino acids: an approach to their manner of action. Acta Horticulturae. 2013. doi:x.17660/ActaHortic.2013.1009.1.
-
Lucini Fifty, Rouphael Y, Cardarelli M, Canaguier R, Kumar P, Colla G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline atmospheric condition. Sci Hortic. 2015;23(182):124–33.
-
Visconti F, De Paz JM, Bonet L, Jordà M, Quiñones A, Intrigliolo DS. Effects of a commercial calcium protein hydrolysate on the salt tolerance of Diospyros kaki L. cv. "Rojo Brillante" grafted on Diospyros lotus L. Sci Hortic. 2015;185:129–38.
-
Del Pilar Cordovilla M, Berrido SI, Ligero F, Lluch C. Rhizobium strain effects on the growth and nitrogen assimilation in Pisum sativum and Vicia faba establish growth under salt stress. J Found Physiol. 1999;154(1):127–31.
-
Rayirath P, Benkel B, Mark Hodges D, Allan-Wojtas P, MacKinnon S, Critchley AT, et al. Lipophilic components of the chocolate-brown seaweed, Ascophyllum nodosum, raise freezing tolerance in Arabidopsis thaliana. Planta. 2009;230(ane):135–47.
-
Nair P, Kandasamy S, Zhang J, Ji 10, Kirby C, Benkel B, et al. Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana. BMC Genom. 2012;xiii(ane):643.
-
Nabati DA, Schmidt RE, Parrish DJ. Consolation of salinity stress in Kentucky bluegrass by plant growth regulators and iron. Crop Sci. 1994;34(1):198–202.
-
Zhang Ten, Ervin EH. Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci. 2008;48(1):364–seventy.
-
Xu C, Leskovar DI. Furnishings of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value nether drought stress. Sci Hortic. 2015;12(183):39–47.
-
Möller M, Smith ML. The significance of the mineral component of seaweed suspensions on lettuce (Lactuca sativa L.) bulb growth. J Institute Physiol. 1998;153(5–6):658–63.
-
Mancuso Southward, Azzarello E, Mugnai S, Briand X. Marine bioactive substances (IPA extract) improve foliar ion uptake and h2o stress tolerance in potted Vitis vinifera plants. Adv Hortic Sci. 2006;20(2):156–61.
-
Elansary HO, Skalicka-Woźniak K, Male monarch IW. Enhancing stress growth traits equally well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol Biochem. 2016;105:310–20.
-
Bradáčová 1000, Weber NF, Morad-Talab Northward, Asim M, Imran Yard, Weinmann M, et al. Micronutrients (Zn/Mn), seaweed extracts, and plant growth-promoting bacteria as cold-stress protectants in maize. Chem Biol Technol Agric. 2016;iii(ane):19.
-
Craigie JS. Seaweed extract stimuli in plant science and agriculture. J Appl Phycol. 2011;23:371–93.
-
Sharma HS, Fleming C, Selby C, Rao JR, Martin T. Found biostimulants: a review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J Appl Phycol. 2014;26:465–90.
-
Verkleij FN. Seaweed extracts in agriculture and horticulture: a review. Biol Agric Hortic. 1992;8:309–24.
-
Khan W, Rayirath Up, Subramanian S, et al. Seaweed extracts equally biostimulants of establish growth and development. J Plant Growth Regul. 2009;28:386–99. doi:10.1007/s00344-009-9103-x.
-
Imran 1000, Mahmood A, Römheld V, Neumann G. Nutrient seed priming improves seedling development of maize exposed to low root zone temperatures during early growth. Eur J Agron. 2013;49:141–8.
-
Guiseley Thousand, Stanley N, Whitehouse P. Carrageenan. Handbook of water-soluble gums and resins. 1980. pp. 5–11.
-
Goñi O, Fort A, Quille P, McKeown PC, Spillane C, O'Connell S. Comparative transcriptome assay of ii Ascophyllum nodosum extract biostimulants: same seaweed but different. J Agric Nutrient Chem. 2016;64(14):2980–ix.
-
de Vasconcelos ACF, Zhang X, Ervin EH, de Kiehl J. C. Enzymatic antioxidant responses to biostimulants in maize and soybean subjected to drought. Scientia Agricola. 2009;66(iii):395–402.
-
Marfà O, Cáceres R, Polo J, Ródena J. Animate being protein hydrolysate equally a biostimulant for transplanted strawberry plants subjected to common cold stress. Acta Horticulturae. 2009. doi:10.17660/ActaHortic.2009.842.57.
-
Szabados L, Savouré A. Proline: a multifunctional amino acrid. Trends Plant Sci. 2010;xv(two):89–97.
-
Nanjo T, Kobayashi M, Yoshiba Y, Sanada Y, Wada G, Tsukaya H, et al. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Establish J. 1999;18(2):185–93.
-
Posmyk MM, Szafrańska K. Biostimulators: a new trend towards solving an quondam trouble. Front Establish Sci. 2016;7:48.
-
Peel GJ, Mickelbart MV, Rhodes D. Choline metabolism in glycinebetaine accumulating and non-accumulating near-isogenic lines of Zea mays and Sorghum bicolor. Phytochemistry. 2010;71(iv):404–xiv.
-
Park E-J, Jeknic Z, Chen THH. Exogenous application of glycinebetaine increases spooky tolerance in love apple plants. Institute Prison cell Physiol. 2006;47(vi):706–fourteen.
-
Chen THH, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;thirteen(ix):499–505.
-
Cuin TA, Shabala S. Exogenously supplied uniform solutes rapidly better NaCl-induced potassium efflux from barley roots. Plant Cell Physiol. 2005;46(12):1924–33.
-
Blum A. Osmotic adjustment is a prime drought stress adaptive engine in back up of plant production. Plant Cell Environ. 2016.
-
Bulgari R, Cocetta M, Trivellini A, Vernieri P, Ferrante A. Biostimulants and ingather responses: a review. Biol Agric Hortic. 2015;31(1):1–17.
-
Zandonadi DB, Santos MP, Caixeta LS, Marinho EB, Peres LEP, Façanha AR, et al. Plant proton pumps as markers of biostimulant action. Scientia Agricola. 2016;73(1):24–8.
-
Selvakumar M, Joshi P, Mishra PK, Bisht JP, Gupta HS. Mountain aspects influence the genetic clustering of psychrotolerant phosphate solubilizing Pseudomonads in the Uttarkhand Himalayas. Curr Microbiol. 2009;59:432–viii.
-
Upadhyay SK, Singh DP. Saikia R Genetic diversity of establish growth promoting rhizobacteria from rhizospheric soil of wheat under saline conditions. Curr Microbiol. 2009;59(5):489–96.
-
Sandhya V, Ali AS, Grover G, Reddy G, Venkateswarlu B. Consolation of drought stress effects in sunflower seedlings past the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils. 2009;46(1):17–26.
-
Zahran HH. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an barren climate. Microbiol Mol Biol Rev. 1999;63(four):968–89.
-
Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CLL, Krishnamurthy L. Establish growth promoting rhizobia: challenges and opportunities. 3 Biotech. 2015;5(4):355–77.
-
Egamberdiyeva D. Consolation of salt stress by establish growth regulators and IAA producing bacteria in wheat. Acta Physiol Constitute. 2009;31(iv):861–4.
-
Paul D, Lade H. Constitute-growth-promoting rhizobacteria to amend crop growth in saline soils: a review. Agron Sustain Dev. 2014;34(4):737–52.
-
Kaushal Thou, Wani SP. Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann Microbiol. 2015;66(1):35–42.
-
Siddiqui KS, Williams TJ, Wilkins D, et al. Psychrophiles. Ann Rev Earth Planet Sci. 2013;41:87–115. doi:ten.1146/annurev-globe-040610-133514.
-
Thao HTB, Yamakawa T. Phosphite (phosphorous acrid): fungicide, fertilizer or bio-stimulator? Soil Sci Establish Nutr. 2009;55(2):228–34.
-
Jiang C, Belfield EJ, Cao Y, Smith JAC, Harberd NP. An arabidopsis soil-salinity–tolerance mutation confers ethylene-mediated enhancement of sodium/potassium homeostasis. Plant Cell. 2013;25(9):3535–52.
-
Nadeem SM, Zahir ZA, Naveed M, Ashraf M. Microbial ACC-deaminase: prospects and applications for inducing common salt tolerance in plants. Crit Rev Constitute Sci. 2010;29(6):360–93.
-
Povero Grand, Mejia JF, Di Tommaso D, Piaggesi A, Warrior P. A systematic approach to discover and narrate natural institute biostimulants. Front Plant Sci [Internet]. 2016;7. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4820456/. Accessed 31 Aug 2016.
-
Rose M, Patti A, Niggling Yard, Brown A, Jackson West, Cavagnaro T. A meta-analysis and review of plant-growth response to humic substances: practical implications for agriculture. Adv Agron. 2014;124:37–89.
Authors' contributions
MJVA and AM wrote the first draft of the manuscript and took care of the revisions of the review; MJVA contributed to the department "Algal Extracts" and "Carbohydrates, proteins, amino acids and lipids"; OP contributed to the department "Microorganisms affecting stress tolerance"; SDP contributed to the section "Carbohydrates, proteins, amino acids and lipids"; SS contributed to the section "Humic and Fulvic Acids" and "Microorganisms affecting stress tolerance". All authors read and approved the last manuscript.
Acknowledgements
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Consent for publication
All authors have given consent for publication.
Ideals blessing and consent to participate
All authors have given consent to participate.
Funding
This work was supported by the Eu Project BIOFECTOR Institute Growth–Promoting Bio-effectors (#FP7-KBBE-2012-half-dozen Grant Understanding 312117) and MACSUR Modelling European Agriculture with Climate change for Food Security—a knowledge hub within FACCE-JPI.
Author information
Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you lot give appropriate credit to the original author(south) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data fabricated available in this commodity, unless otherwise stated.
Reprints and Permissions
Nearly this article
Cite this article
Van Oosten, 1000.J., Pepe, O., De Pascale, S. et al. The part of biostimulants and bioeffectors every bit alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 4, 5 (2017). https://doi.org/x.1186/s40538-017-0089-5
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s40538-017-0089-5
Keywords
- Abiotic stress
- Biostimulants
- Bioeffectors
- Microbial inoculants
- Humic acrid
- Fulvic acid
- Protein hydrolysates
- Amino acids
- Seaweed extracts
- Bioprotection
Source: https://chembioagro.springeropen.com/articles/10.1186/s40538-017-0089-5
Post a Comment for "Can Abiotic Stresses in Plants Be Alleviated by Manganese Nanoparticles or Compounds?"